
Need For Pure Water
 The Company is engaged in diverse Business Activities headed by well-experienced engineers and highly skilled workers in their relevant fields.....
The Company is engaged in diverse Business Activities headed by well-experienced engineers and highly skilled workers in their relevant fields.....Working Principle
Article of Water Quality Association
The article below is provided by the Water Quality Association
What is Reverse Osmosis?
Anyone who has been through a high school science class will likely be familiar with the term osmosis. The process was first described by a French Scientist in 1748, who noted that water spontaneously diffused through a pig bladder membrane into alcohol. Over 200 years later, a modification of this process known as reverse osmosis allows people throughout the world to affordably convert undesireable water into water that is virtually free of health or aesthetic contaminants. Reverse osmosis systems can be found providing treated water from the kitchen counter in a private residence to installations used in manned spacecraft.
Reverse Osmosis is a technology that is found virutally anywhere pure water is needed; common uses include:
How Reverse Osmosis Works
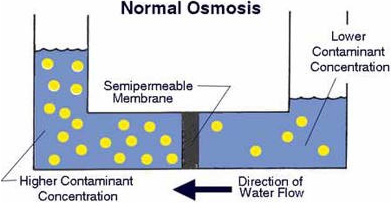
A semipermeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water i the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and an equilibrium will form.
What is Reverse Osmosis?
Anyone who has been through a high school science class will likely be familiar with the term osmosis. The process was first described by a French Scientist in 1748, who noted that water spontaneously diffused through a pig bladder membrane into alcohol. Over 200 years later, a modification of this process known as reverse osmosis allows people throughout the world to affordably convert undesireable water into water that is virtually free of health or aesthetic contaminants. Reverse osmosis systems can be found providing treated water from the kitchen counter in a private residence to installations used in manned spacecraft.
Reverse Osmosis is a technology that is found virutally anywhere pure water is needed; common uses include:
Drinking Water |
Pharmaceutical Production |
Humidification |
Kidney Dialysis |
Ice-Making |
Water used in chemcial processes |
Car Wash Water Reclamation |
Cosmetics |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
Drinking Water |
How Reverse Osmosis Works
A semipermeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water i the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and an equilibrium will form.

|
